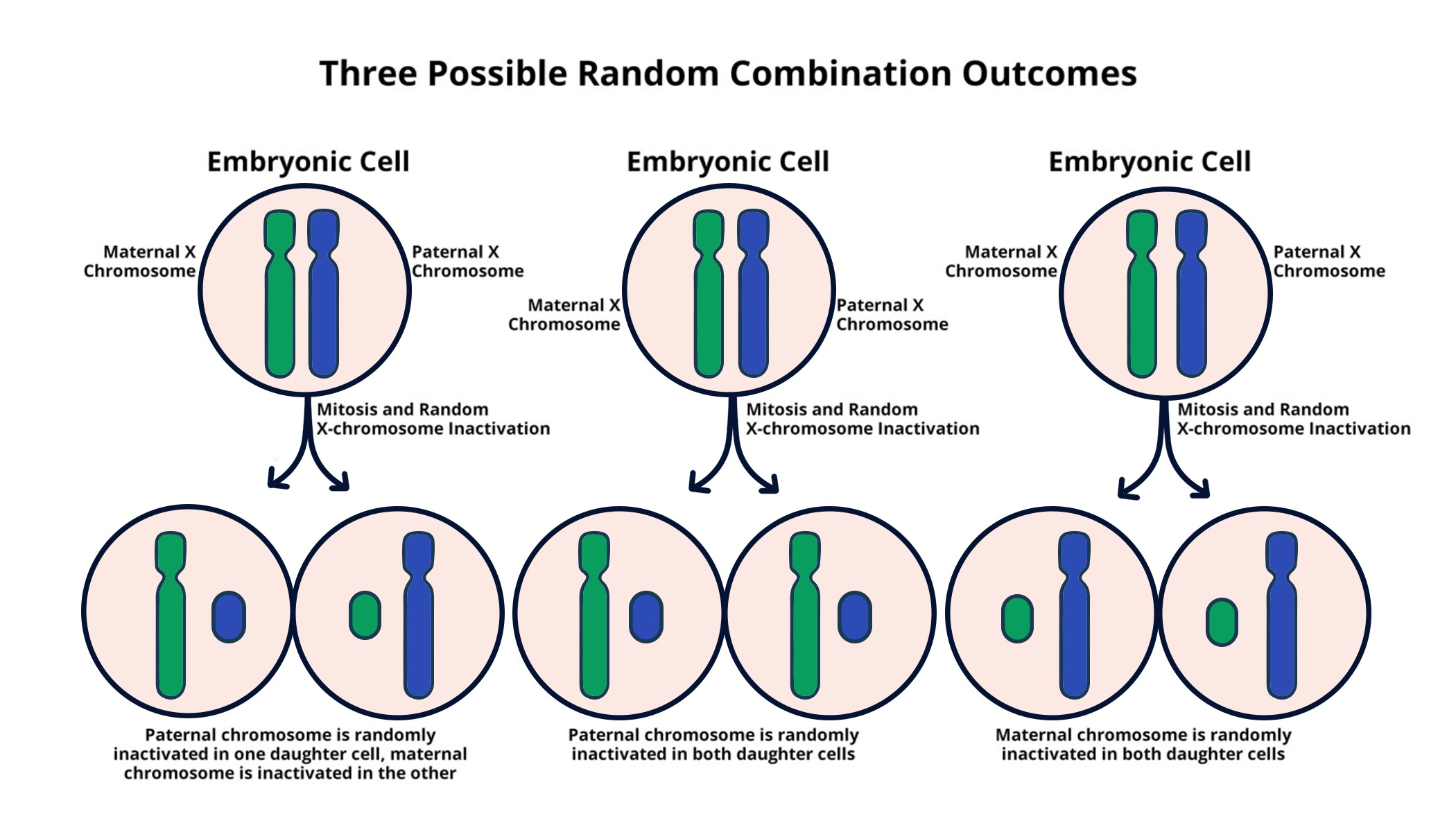
X chromosome inactivation is a vital biological process that addresses the inherent discrepancy in X chromosome dosage between males and females. In females, where two X chromosomes are present, one must be inactivated to prevent an overexpression of genes, creating an intriguing area of study within chromosomal research. This complex mechanism provides insights into genetic disorders such as Fragile X Syndrome and Rett Syndrome, both of which arise from mutations on the X chromosome. Recent findings suggest that understanding how this inactivation works not only sheds light on fundamental cellular biology but also opens doors for potential therapeutic interventions. As scientists like Jeannie Lee tackle these fundamental questions, the implications for treating these and other genetic disorders are becoming increasingly promising.
The phenomenon of X chromosome inactivation, also known as Lyonization, plays a crucial role in gene expression regulation in human biology. In essence, this process serves as a mechanism for balancing gene dosage by silencing one of the two X chromosomes found in females. Researchers have focused on this critical silencing phenomenon to better understand its relationship with various genetic conditions, including notable disorders like Fragile X and Rett Syndrome. By dissecting the complexities of this chromosomal event, scientists hope to unravel potential therapies, targeting the silent genes to restore functionality. The progress in this field highlights the intersection of genetics and medicine, illustrating how basic research can lead to breakthroughs in treating chromosomal anomalies and enhancing patient outcomes.
Understanding X Chromosome Inactivation: A Cellular Mystery
The X chromosome is unique within the human genome due to its role in gene dosage compensation, particularly in females who possess two copies. This exceptional requirement leads to the biological phenomenon known as X chromosome inactivation (XCI), where one of the X chromosomes is randomly silenced to balance the expression of X-linked genes between males and females. Initially discovered in female mammals, this process has sparked significant interest in cellular biology because it provides insights into how organisms manage gene expression and prevent disorders linked to chromosomal imbalances. The intricacies of XCI, including the role of specific RNA molecules like Xist, have been the focus of extensive research, revealing the complex mechanisms that underlie this critical cellular function.
Through the lens of chromosomal research, understanding X chromosome inactivation has far-reaching implications. Not only does it help elucidate fundamental biological processes, but it also sheds light on the pathology of various genetic disorders associated with the X chromosome, including Fragile X Syndrome and Rett Syndrome. By uncovering how inactivation occurs, scientists can explore potential therapeutic avenues for reversing this process in individuals affected by these conditions. Thus, investigating the nuances of XCI is not just an academic endeavor; it holds the promise of advancing treatments for a range of genetic disorders and improving patient outcomes.
Implications of X Chromosome Inactivation on Genetic Disorders
The breakthroughs in understanding X chromosome inactivation by researchers, notably Jeannie Lee’s team, carry transformative implications for the treatment of genetic disorders. In conditions such as Fragile X Syndrome and Rett Syndrome, mutations often reside on one of the two X chromosomes in affected individuals. By successfully unlocking the inactivated X chromosome, scientists aim to access healthy versions of these genes that would otherwise remain dormant. This rejuvenation of gene expression could potentially address the root causes of these serious conditions, offering hope to those who are currently faced with limited therapeutic options.
Moreover, the discovery that X chromosome inactivation mechanisms can be manipulated opens the door to innovative treatment strategies targeting various genetic disorders. Researchers are particularly excited about the applicability of their findings beyond females; preliminary insights suggest that even males could benefit from therapies that exploit these mechanisms. By understanding the biophysical properties of the gelatinous substance that facilitates XCI, scientists can develop targeted therapies that resensitize mutated genes while preserving the integrity of unaffected genes. As such, ongoing research in chromosomal biology promises not only to unravel genetic mysteries but also to pave the way for effective interventions against debilitating disorders linked to the X chromosome.
Exploring Treatments for Fragile X Syndrome and Rett Syndrome
The journey toward developing effective treatments for Fragile X Syndrome and Rett Syndrome is rooted in groundbreaking research on X chromosome inactivation. Scientists, including those in Jeannie Lee’s lab, are looking to dissect the pathways that regulate gene expression in these conditions. Since these syndromes are directly associated with mutations on the X chromosome, understanding how to unsilence the inactivated chromosome holds the key to potential therapies. Current investigations focus on optimizing compounds that can reverse XCI in controlled laboratory settings, which could lead to promising clinical applications.
Not only does this research carry the potential to directly benefit individuals suffering from Fragile X Syndrome and Rett Syndrome, but it also illustrates the broader implications of chromosomal research in medical science. As researchers refine their methodologies, there is hope that such interventions could eventually be generalized to treat a variety of genetic disorders stemming from similar X-linked mutations. By advancing the field of genetic therapies, these scientists are poised to redefine the therapeutic landscape, transforming the lives of countless patients and paving the way for future advancements.
The Role of Xist RNA in Chromosome Silencing
Central to the process of X chromosome inactivation is the Xist RNA molecule, which plays a pivotal role in both the initiation and maintenance of chromosomal silencing. This long non-coding RNA regulates chromatin modifications and influences the physical state of the surrounding genomic environment. When Xist is transcribed, it binds to the X chromosome, promoting changes that lead to its silencing. Understanding the specific interactions between Xist and the chromatin architecture has become a key focus for scientists aiming to unravel the complexities of XCI.
Research into Xist RNA not only contributes to our fundamental understanding of cellular mechanisms but also unveils potential therapeutic targets for genetic conditions related to X chromosome mutations. By exploring how Xist modulates gene expression, researchers can develop strategies aimed at harnessing its properties to reactivate silenced genes in affected individuals. This work holds promise not only for addressing Fragile X Syndrome and Rett Syndrome but also for a wider array of genetic disorders stemming from the intricacies of X-linked gene regulation.
Historical Perspectives on X Chromosome Research
The history of X chromosome research is as intricate as the chromosome itself. Over the past few decades, scientists have made significant strides in understanding the biological processes underpinning X chromosome inactivation. Institutional funding, particularly from the National Institutes of Health, has been critical in supporting pioneering research in this field. Initially, researchers like Jeannie Lee were dedicated to answering fundamental questions about the mechanisms of XCI. The transition from basic research to therapeutic application showcases the evolution of our understanding of gene regulation on the X chromosome.
Looking back, the revelations surrounding X chromosome inactivation balance the historical narrative of scientific discovery with contemporary medical advancements. Each breakthrough and subsequent study has chipped away at prevalent mysteries, illustrating the importance of persistent inquiry in genetics. As we continue to explore the ramifications of X-linked genetic disorders, the foundational knowledge gleaned from decades of research serves as a springboard for future innovations in treatment and understanding of these complex chromosomal phenomena.
The Future of Genetic Therapies Targeting Chromosomal Disorders
The future of genetic therapies targeting disorders linked to the X chromosome looks promising, buoyed by the latest discoveries in chromosomal biology. With a better understanding of X chromosome inactivation and the molecular mechanisms that govern it, researchers are optimistic about the development of innovative treatments. The clinical potential of reactivating silenced genes presents a new frontier in gene therapy, aiming to correct or compensate for genetic disorders such as Fragile X Syndrome and Rett Syndrome. This approach emphasizes a shift towards personalized medicine, where therapies are tailored to address specific genetic mutations.
As science progresses, the ongoing refinement of methods to manipulate the inactivated X chromosome may lead to breakthroughs that improve quality of life for those affected by genetic disorders. Researchers are currently focused on conducting rigorous safety studies to ensure that newly developed compounds can be transitioned into clinical trials successfully. Furthermore, by exploring the relationship between gene expression and chromosomal architecture, future therapies may also provide insights into managing other genetic conditions. The potential to unlock the therapeutic capabilities of the X chromosome heralds an exciting era in the battle against genetic disorders.
Challenges in Unraveling Chromosomal Mechanisms
Despite the substantial advancements made in understanding X chromosome inactivation and its implications for genetic disorders, significant challenges remain. One of the primary complexities is investigating how specific genes remain unaffected while others are restored to functionality after unsilencing. This paradox raises questions about the cellular mechanisms governing gene priorities and the limits of gene expression capacity. Jeannie Lee’s ongoing research aims to address these challenging dynamics to better understand the selective reactivation of mutated genes.
Additionally, the task of translating laboratory discoveries into effective clinical treatments is fraught with hurdles. Ensuring that any therapeutic interventions maintain the integrity of unwounded genes while correcting mutations poses a technical challenge. This calls for a multidisciplinary approach as researchers collaborate to understand the interplay between gene silencing, gene expression, and chromosomal architecture. As they navigate these intricate relationships, the ultimate goal remains clear: to provide updated and effective treatment options for individuals affected by X-linked genetic disorders.
Public Awareness of Genetic Disorders Linked to the X Chromosome
Public awareness of genetic disorders such as Fragile X Syndrome and Rett Syndrome is crucial in fostering action towards research and potential funding for genetic therapies. Many people remain unaware of the implications of X chromosome inactivation and its association with these conditions, which can lead to significant challenges for affected families. Therefore, educational efforts that communicate the genetic basis of these disorders and ongoing research are vital for generating community support and understanding.
Raising awareness also empowers families to seek genetic testing and make informed decisions regarding treatment options. Through advocacy and dissemination of knowledge about X-linked genetic disorders, communities can better support individuals affected by these conditions. Furthermore, increased public awareness can drive funding and resources toward research efforts, ultimately propelling the scientific community closer to effective therapies. By highlighting the importance of tackling genetic disorders at the chromosomal level, we encourage a societal commitment to improving health outcomes for those impacted by X chromosome anomalies.
Frequently Asked Questions
What is X chromosome inactivation and why is it important?
X chromosome inactivation (XCI) is a biological process that occurs in females, where one of the two X chromosomes is randomly inactivated to ensure that males and females have equal expression of X-linked genes. This process is crucial because it prevents an excess of gene products stemming from the genes located on the X chromosome, thus maintaining cellular balance in females.
How does X chromosome inactivation relate to genetic disorders like Fragile X Syndrome and Rett Syndrome?
X chromosome inactivation is particularly relevant to genetic disorders such as Fragile X Syndrome and Rett Syndrome, which are caused by mutations on the X chromosome. In these disorders, the healthy gene on the inactivated X chromosome is often unavailable for use, leading to the onset of disease symptoms. Targeting XCI to unsilence the healthy gene may provide therapeutic benefits for individuals with these conditions.
What role does the Xist RNA molecule play in X chromosome inactivation?
The Xist RNA molecule is pivotal in the process of X chromosome inactivation. It is produced by a gene on the X chromosome and binds to the chromosome, altering the surrounding chromosomal material’s properties. This interaction facilitates the silencing of one X chromosome by modifying its structure, thus playing a critical role in maintaining the balance of X-linked gene expression.
Can X chromosome inactivation be manipulated to treat genetic disorders?
Yes, researchers are exploring ways to manipulate X chromosome inactivation to treat genetic disorders. Recent studies have shown that it is possible to unsilence the inactive X chromosome and restore the expression of healthy genes, potentially providing a therapeutic avenue for conditions like Fragile X Syndrome and Rett Syndrome.
What challenges remain in understanding X chromosome inactivation?
Despite significant advances, challenges remain in fully understanding X chromosome inactivation. Researchers are still investigating why some genes on the X chromosome remain unaffected during the unsilencing process, and how to optimize this strategy for therapeutic use, ensuring minimal side effects while maximizing the expression of healthy genes.
How does X chromosome inactivation affect males compared to females?
Males typically have only one X chromosome, so they do not undergo X chromosome inactivation like females. However, they can still experience gene silencing at specific points if mutations are present. This suggests that similar mechanisms may operate in male cells regarding X-linked disorders, albeit without the dual X chromosome context.
What future implications does research on X chromosome inactivation have for chromosomal research?
The ongoing research into X chromosome inactivation has significant implications for chromosomal research, as it could lead to breakthroughs in treating various genetic disorders. By understanding how to modulate this process, researchers hope to develop targeted therapies that could restore functionality to mutations found on the X chromosome, opening new pathways for treatment.
| Key Points |
|---|
| Females have two X chromosomes while males have one, necessitating X chromosome inactivation in females. |
| X chromosome inactivation involves a gelatinous substance that separates chromosomes, compared to Jell-O, preventing tangling. |
| The gene Xist plays a crucial role by changing the properties of the surrounding substance and helping inactivate the X chromosome. |
| Potential treatments are being developed for genetic disorders caused by X-linked mutations, including Fragile X and Rett Syndrome. |
| The research provides insights into freeing inactivated X chromosomes, potentially restoring gene function with minimal side effects. |
Summary
X chromosome inactivation is a vital biological process that allows females to manage their two X chromosome copies effectively. This study illustrates significant advances in understanding how X chromosome inactivation operates, revealing insights that could lead to new therapeutic strategies for disorders stemming from mutations on the X chromosome. The work accomplished by Jeannie T. Lee’s lab highlights the delicate balance of gene regulation and presents promising directions for future treatments of diseases such as Fragile X syndrome and Rett syndrome. As research continues, the potential to safely reactivate genes on the inactivated X chromosome could revolutionize the management of these genetic disorders.