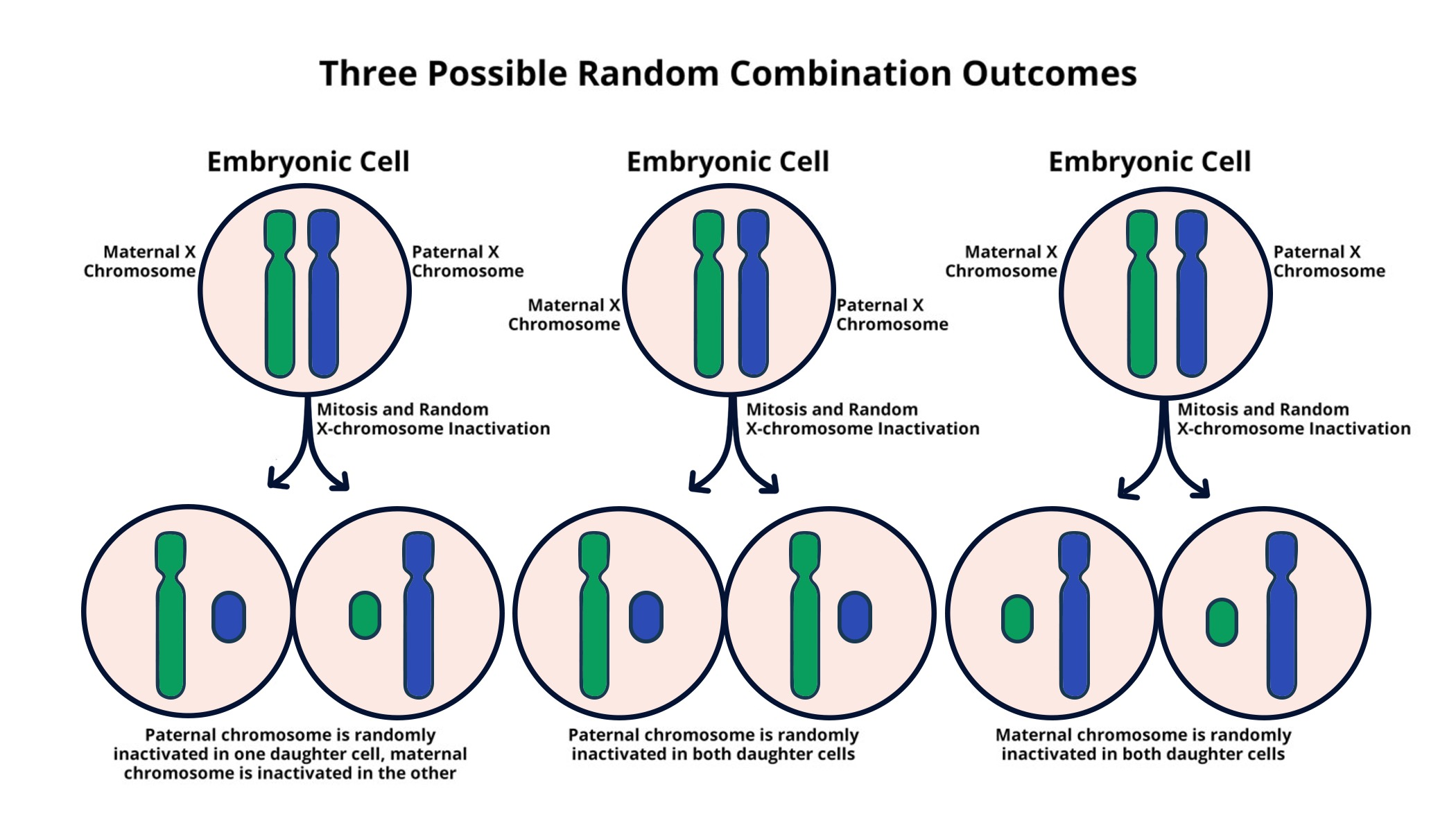
X chromosome inactivation is a fascinating biological process that serves as a critical mechanism of gene regulation in females. Unlike males, who possess only one copy of the X chromosome, females have two, necessitating the silencing of one to avoid double dosing of X-linked genes. This intricate process has been the focus of extensive chromosomal research, especially with respect to its implications in genetic diseases such as Fragile X Syndrome and Rett Syndrome. Recent studies, particularly from Jeannie T. Lee’s lab, reveal that understanding X chromosome inactivation could pave the way for innovative gene therapy approaches to tackle these disorders. As scientists delve deeper into the mechanism behind this silencing, the potential for therapeutic breakthroughs becomes increasingly promising.
The inactivation of the X chromosome, often referred to as XCI, is a vital process that ensures balanced gene expression between genders. In females, with two X chromosomes, one is intentionally silenced, creating a functional equivalent to the male’s single X chromosome. This phenomenon is particularly significant in the context of certain genetic disorders linked to the X chromosome, including conditions like Fragile X Syndrome and Rett Syndrome. Ongoing chromosomal investigations aim to uncover the mysteries of this complex mechanism, which may ultimately lead to novel gene therapy interventions. By illuminating the pathways of X chromosome silencing, researchers hope to unlock new treatments for those affected by related genetic diseases.
Understanding X Chromosome Inactivation and its Importance
X chromosome inactivation (XCI) is a crucial biological process that occurs in female mammals, where one of the two X chromosomes is randomly silenced to ensure dosage compensation between the sexes. This means that females, who possess two copies of the X chromosome, do not express twice the number of X-linked genes compared to males, who have only one. The intricate mechanism of XCI serves not only to maintain genetic balance but also plays a significant role in the onset of various genetic diseases linked to mutations on the X chromosome. Understanding how XCI is regulated can pave the way for innovative treatments for conditions such as Fragile X Syndrome and Rett Syndrome, both of which are caused by abnormalities in genes located on the X chromosome.
Recent research spearheaded by scientists, including those in Jeannie Lee’s lab, has shed light on the mechanistic details of X chromosome inactivation. This process involves a unique RNA molecule called Xist, which coats the X chromosome, turning it into a silent entity within the cell. The interaction of Xist with the surrounding chromosomal structure, often described as a Jell-O-like substance, plays a key role in the silencing process. By elucidating the biological underpinnings of XCI, researchers can explore targeted gene therapies that potentially reactivate dormant genes on the inactivated X chromosome, thereby addressing the root causes of specific genetic disorders.
Breakthroughs in Treating Genetic Diseases through XCI Research
As foundational research on X chromosome inactivation progresses, the potential therapeutic implications for genetic diseases grow increasingly promising. Scientists have discovered methods to manipulate this silencing process, aiming to unsilence the X-linked genes that are crucial for normal cellular function but are rendered inactive due to XCI. The implications are particularly significant for individuals affected by conditions like Fragile X Syndrome, a genetic disorder caused by the expansion of fragile sites on the X chromosome, leading to intellectual disabilities and developmental delays. If researchers can successfully reactivate the healthy gene form found on the inactivated X chromosome, it could offer a revolutionary approach to treating Fragile X Syndrome.
Furthermore, the insights gained from studying X chromosome inactivation may extend to Rett Syndrome, another X-linked disorder characterized by neurological development issues primarily affecting females. By targeting the molecular mechanisms governing XCI, researchers can focus on gene therapy strategies that aim to rectify these genetic mutations. This approach has far-reaching potential not just for females, who generally inherit one affected X chromosome, but also for males who might carry specific mutations leading to similar effects. The drive for further optimization of these methods and the transition into clinical trials can provide hope for thousands currently living with these disabilities.
The Role of Chromosomal Research in Advancing Gene Therapy
Chromosomal research plays an essential role in the field of genetics, particularly in improving our understanding of complex genetic diseases. By exploring the dynamics of chromosome behavior, such as the mechanism of X chromosome inactivation, researchers can unveil the genetic basis of various disorders. Areas like chromosomal architecture and gene interactions are critical to developing new therapeutic strategies aimed at gene therapy. As the understanding of chromosomal structures improves, so do the methodologies to target gene expressions in specific conditions, including those linked to the X chromosome.
Innovative approaches derived from chromosomal research can enhance the efficacy of gene therapies. By understanding how genes are expressed or repressed within their chromosomal contexts, scientists can create more precise treatments that minimize potential side effects. The momentum generated by studies on XCI highlights the promising future of gene therapy, especially for conditions like Fragile X Syndrome and Rett Syndrome, where specific gene reactivation could elicit substantial improvements in patient outcomes. As research evolves, the hope is that tailored gene therapies will emerge as viable options providing relief for individuals affected by these challenging genetic disorders.
Implications of XCI Discoveries on Future Treatments
The discovery of mechanisms behind X chromosome inactivation has significant implications for developing targeted therapies for genetic diseases. As researchers uncover how to manipulate the silencing of the X chromosome, the prospect of freeing the inactivated chromosomes to restore healthy gene function becomes more tangible. This has the potential to revolutionize treatment approaches for disorders such as Fragile X Syndrome and Rett Syndrome, where only a fraction of affected individuals are able to access the healthy genes that are involved in these conditions. The long-term aim is to transition these basic research insights into practical therapies that can significantly enhance the quality of life for affected individuals.
Moreover, the ability to reactivate genes on inactivated X chromosomes could lead to breakthroughs not just in understanding but also in treating broader genetic diseases. It raises the possibility of utilizing gene therapy not merely for replacement but also for restoration of gene function, making it a critical avenue for ongoing research. As scientists like Jeannie Lee and her team refine their approaches and conduct safety studies, the potential for clinical trials to introduce these findings into treatment regimens could be on the horizon, bringing hope to thousands grappling with the consequences of genetic diseases.
Challenges Facing XCI Research and Gene Therapy
Despite the promising advancements in understanding X chromosome inactivation, numerous challenges remain that could impede the translation of these insights into effective gene therapies. One significant challenge is ensuring that treatments effectively target only the mutated genes while sparing healthy ones, which is crucial to minimize unintended consequences. Researchers need to unravel the precise regulatory mechanisms involved in XCI to avoid reactivating merely any gene. Understanding the interplay between genes and their chromosomal environments continues to be a complicated yet essential task in ensuring safe and effective gene therapy interventions.
There is also the challenge of individual variability in genetic diseases. Each patient may exhibit different mutations or combinations of mutations on the X chromosome, which necessitates a tailored approach to treatment. As gene therapy strategies become more sophisticated, they must also account for these variations in genetic expression and chromosomal structure. As researchers embark on creating treatments, they must incorporate robust methodologies to not only address the specific mutations associated with diseases like Fragile X Syndrome and Rett Syndrome but also to monitor and evaluate the possible side effects rigorously.
Future Directions in Chromosomal Research and Health
The future of chromosomal research holds immense potential in unraveling further insights into genetic diseases and their treatments. As technologies improve, techniques such as CRISPR and other gene-editing tools are providing new avenues to explore the specifics of chromosome function and gene regulation. This expansion of genetic research will continue to incentivize developing treatments not only for X-linked disorders but for a broader range of genetic conditions. The ongoing investigations into X chromosome inactivation will likely yield important information that can be applied to optimize gene therapy techniques.
Additionally, interdisciplinary collaboration among geneticists, biologists, and medical professionals can expedite the translation of research findings into clinical applications. Stakeholders must work together to pull from each field’s advancements, creating innovative pathways for therapy development. This collaborative effort will be essential in establishing targeted approaches that align with the personalized medicine paradigm, providing tailored solutions to patients based on their unique genetic profiles. As the translational research field pushes forward, the hope is that discoveries from XCI studies will blossom into accessible treatments for many adversities faced by individuals suffering from genetic diseases.
Community Impact of Genetic Diseases and Awareness
The impact of genetic diseases, particularly those linked to the X chromosome such as Fragile X Syndrome and Rett Syndrome, extends beyond the individual to affect families and communities. Raising awareness about these conditions is paramount for fostering understanding, acceptance, and support for affected individuals. Many families affected by X-linked disorders often face emotional and financial challenges, making community support systems a critical aspect of coping and resilience. By promoting discussions about genetic diseases within the community, we can create a pathway for improved resources and support networks.
Moreover, awareness campaigns can help highlight the importance of ongoing research, funding, and advocacy for genetic diseases. Communities play a vital role in supporting initiatives that aim to fund research, raise consciousness about genetic disorders, and potentially aid in the development of therapies. Engaging local stakeholders can help encourage collaborative efforts that align with research objectives, including the push toward clinical trials based on findings from studies on X chromosome inactivation. Building this awareness can foster alignment that ultimately leads to improved outcomes for those affected by genetic diseases.
The Role of Public Funding in Genetic Research
Public funding has been a cornerstone in advancing genetic research, particularly in exploring the complexities of X chromosome inactivation and its implications for treating genetic diseases. Support from institutions such as the National Institutes of Health has fueled essential research endeavors focused on understanding the genetic mechanisms underlying disorders like Fragile X Syndrome and Rett Syndrome. Such funding activities enable researchers to dive deep into foundational questions while also facilitating the development of potential therapies that could alleviate the burdens of these conditions.
As research continues to evolve, the allocation of public funding is critical to sustaining the momentum needed for breakthroughs in gene therapy and chromosomal studies. Advocacy for continued investment in genetic research is necessary, as it can lead to transformative therapies that make a meaningful impact on patients’ lives. The collaboration between public agencies and research institutions can create a robust framework for innovation, increasing the chances of translating scientific findings into effective treatments for genetic diseases linked to the X chromosome.
Frequently Asked Questions
What is X chromosome inactivation and why is it important in genetics?
X chromosome inactivation (XCI) is a biological process where one of the two X chromosomes in females is randomly inactivated to prevent overexpression of X-linked genes. This process is crucial for ensuring balanced gene dosage between males (who have one X chromosome) and females (who have two). Abnormalities in XCI can lead to genetic diseases such as Fragile X Syndrome and Rett Syndrome, highlighting its significance in chromosomal research.
How does X chromosome inactivation relate to genetic diseases like Fragile X Syndrome?
Fragile X Syndrome is a genetic disorder linked to mutations on the X chromosome. In females, X chromosome inactivation can silence the affected X chromosome, making the healthy gene on the other X chromosome critical for normal development. Understanding XCI helps researchers develop gene therapies aimed at reversing this silencing, potentially offering treatments for Fragile X Syndrome.
What role does Xist RNA play in X chromosome inactivation?
Xist RNA is a critical component of the X chromosome inactivation process. It coats the X chromosome, altering its interaction with surrounding nucleoplasm, which helps to trigger the inactivation process. This mechanism is essential for silencing one X chromosome in females, and research into Xist could lead to advancements in treating genetic diseases linked to the X chromosome.
How might advances in understanding X chromosome inactivation lead to therapies for Rett Syndrome?
Research into X chromosome inactivation has unveiled how silenced genes can be unlocked, particularly in cases of Rett Syndrome, which is also caused by mutations on the X chromosome. By developing therapies that target the mechanisms of XCI, scientists hope to restore function to mutated genes in individuals with Rett Syndrome, potentially alleviating symptoms and improving quality of life.
Could gene therapy be a solution for genetic diseases caused by X chromosome mutations?
Yes, gene therapy is a promising avenue for addressing genetic diseases caused by mutations on the X chromosome, such as Fragile X Syndrome and Rett Syndrome. By modifying X chromosome inactivation mechanisms and unsilencing affected genes, researchers aim to create therapies that can restore normal function without disrupting healthy gene expression, providing hope for effective treatments.
What are the future prospects for therapies targeting X chromosome inactivation?
The future of therapies targeting X chromosome inactivation is promising, with ongoing research focused on unsilencing inactivated genes. As scientists optimize approaches developed from understanding XCI, such as those for Fragile X Syndrome and Rett Syndrome, we may soon see the transition to clinical trials, promising new options for those affected by genetic disorders linked to the X chromosome.
| Key Point | Description |
|---|---|
| X Chromosome Inactivation | Females have two X chromosomes which they must inactivate one for proper gene dosage, unlike males who have one. |
| Role of Xist | Xist RNA plays a crucial role in the inactivation process by altering the surrounding ‘Jell-O’ substance. |
| Chromosomal Jell-O | A gel-like substance organizes chromosomes and is critical for separating structures to prevent entanglement. |
| Therapeutic Potential | Understanding X chromosome inactivation can lead to treatments for genetic disorders like Fragile X and Rett syndromes. |
| Research Progress | After decades of research, new techniques to ‘unsilence’ inactivated X-linked genes are being developed. |
| Future Research | Ongoing studies will focus on optimizing treatments and conducting safety evaluations for clinical trials. |
Summary
X chromosome inactivation is a vital biological mechanism that resolves the discrepancy in X-linked gene dosage between females and males. Through the innovative research led by Jeannie T. Lee at Harvard Medical School, the complexities of how cells inactivate one of the two X chromosomes in females have begun to unravel. This work opens avenues for potential therapies targeting genetic diseases associated with the X chromosome, such as Fragile X syndrome and Rett syndrome, by mastering the mechanism of unsilencing inactivated genes. As the scientific community delves deeper into X chromosome inactivation, the hope grows for effective treatments that could alleviate the burdens of these genetic disorders.